Do you know that you can save money every day from the service you like or the items you will purchase? I have summarized all the savings ideas in one single post. Please check out how much you can save today.
 |  |
Basic Properties of Elemental Zinc
| Group | 12 |
| Period | 4 |
| Block | d |
| Atomic number | 30 |
| State at 20°C | Solid |
| Electron configuration | [Ar] 3d104s2 |
| Melting point: | 419.53 °C |
| Boiling point: | 907°C |
Zinc Elements
Ø Fourth most widely consumed metal
Ø Zinc compounds have been used for at least 2,500 years as brass
Ø Metallic zinc was first produced in India sometime in the 1400s
Ø German chemist Andreas Marggraf isolated the element in 1746
Ø Today, most zinc is produced through the electrolysis of aqueous zinc sulfate
Most Abundant Isotopes of Zinc
| 64Zn | 49.2% |
| 66Zn | 27.7% |
| 67Zn | 4.0% |
| 68Zn | 18.5% |
Standard atomic weight of Zinc: 65.38
Zinc Reserves by Country
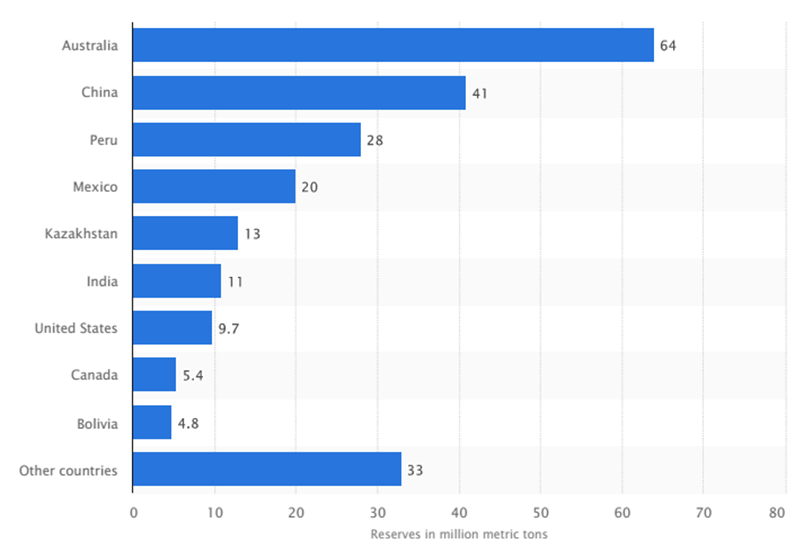
Zinc Mining and Production by Country
| Rank | Country | Mine production in 2017 |
| 1 | China | 5.1 million MT |
| 2 | Peru | 1.4 million MT |
| 3 | India | 1.3 million MT |
| 4 | Australia | 1 million MT |
| 5 | United States | 730,000 MT |
| 6 | Mexico | 680,000 MT |
| 7 | Bolivia | 500,000 MT |
| 8 | Kazakhstan | 360,000 MT |
| 9 | Canada | 340,000 MT |
| 10 | Sweden | 260,000 MT |
Production of Zinc by the Miners

| Red Dog § World’s largest zinc mine § Located in Alaska § Accessible only by air or barge during the summer. § Open-pit truck-and-loader operation § Highest grades of zinc in reserves among top zinc producers. |  |
Electrolytic Process of Zinc Mining
The process has four stages:
a) concentration of the ore
b) roasting of the ore in air
c) conversion of zinc oxide to zinc sulfate
d) electrolysis of zinc sulfate solution
(a) Concentration of the ore
Ø The ore is mined, crushed, ball-milled and then concentrated by froth flotation.
Ø This removes unwanted components, including the lead compounds and waste rock
(b) Roasting of the ore in air
Ø Takes place in a furnace at around 1300 K
Ø The most important reaction is the conversion of zinc sulfide to zinc oxide. Sulfur dioxide is often converted to sulfuric acid.

(c) Conversion of zinc oxide to zinc sulfate
The crude zinc oxide is leached in sulfuric acid to dissolve the oxide as zinc sulfate

(d) Electrolysis of zinc sulfate solution
Ø Zinc is liberated preferentially at the cathodes
Ø Every 24 to 72 hours zinc is stripped off the electrodes, melted and cast into ingots.
Ø The metal is at least 99.96% pure.
Uses of Zinc
- Zinc is used to galvanize other metals to prevent rusting Galvanized steel is used for car bodies, street lamp posts, safety barriers and suspension bridges
- Zinc is used in different types of batteries
- Zinc is used to produce die-castings, which are important in the automobile, electrical and hardware industries
- Zinc is also used in alloys such as brass, nickel silver and aluminium solder
- Zinc sulfide is used in making luminous paints, fluorescent lights and x-ray screens
- Zinc oxide is widely used in the manufacture of paints, rubber, cosmetics, pharmaceuticals, plastics, inks, soaps, batteries, textiles and electrical equipment
Uses of Zinc in Energy
- Zinc–carbon battery
- Alkaline battery
- Zinc–air battery
- Zinc chloride battery
- Zinc–bromine battery
- Zinc–cerium battery
- Nickel–zinc battery
- Silver-zinc battery
- Zinc ion battery
Zinc Carbon Battery
| First commercial dry batteries Anode: Zinc Cathode: Carbon Electrolyte: ammonium chloride and manganese dioxide |  |
Anode: Zn(s) → Zn2+(aq) + 2 e−
Cathode: 2 MnO2(s) + 2 e− + 2 NH4Cl(aq) → Mn2O3(s) + 2 NH3(aq) +
H2O(l) + 2 Cl−(aq)
Cell Voltage: 1.5 V
Uses: Clocks, flashlights, remote controls, portable radios
Alkaline Batteries
| Cathode: Paste of manganese dioxide with carbon powder Anode: Zinc powder in a gel containing the potassium hydroxide Separator: Non-woven layer of cellulose or a synthetic polymer Common Size: AAA, AA, C, sub-C and D | 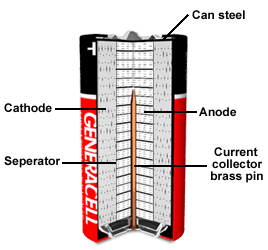 |
Anode: Zn(s) + 2OH−(aq) → ZnO(s) + H2O(l) + 2e−
Cathode: 2MnO2(s) + H2O(l) + 2e− → Mn2O3(s) + 2OH−(aq)
Cell reaction: Zn(s) + 2MnO2(s) ⇌ ZnO(s) + Mn2O3(s)
Cell Voltage: 1.5 V
Uses: Practically most portable devices
Zinc Air Batteries
Larger energy density at lower production cost
Anode: Zinc
Cathode: Oxygen from the air
Uses: Hearing aids, electric vehicle propulsion, navigation instruments, marker lights, oceanographic experiments, railway signals
Anode: Zn + 4OH− → Zn(OH)42− + 2e−
Fluid: Zn(OH)42− → ZnO + H2O + 2OH−
Cathode: 1/2 O2 + H2O + 2e− → 2OH−
Overall: 2Zn + O2 → 2ZnO
Cell Voltage: 1.59 V
Rechargeable Zinc Air Batteries
- Cheaper than lithium ion batteries. Lithium ion batteries costs $250 per kilowatt-hour whereas, rechargeable zinc air batteries cost $100 per kilowatt-hour
- NantEnergy is the true leader in rechargeable zinc air batteries
- Produce self-contained solar-power batteries
- Primary focus of the company is microgrids
- Serve small areas rather than individual residential customers
- Future goal : Transportation systems like electric cars, buses, trains and scooters
Achievement of NantEnergy
§ Over 1,000 Deployed Sites in 9 Countries
§ Deployed in Over 100 Villages in Africa & Asia
§ Deployed in Over 1,000 Cell Tower Sites Worldwide
§ Over 3,000 Systems of 40 Cells Per System Globally
§ Broke the $100 kWh Manufacturing Cost Barrier
§ Sole Source of Power to 200,000 People
Nickel Zinc Batteries
Thomas Alva Edison invented in 1901†
Anode: Zn + 4 OH− ↔ Zn(OH)42− + 2e−
Electrolyte: KOH + Zn(OH)42− ↔ Zn(OH)2 + 2OH−
Zn(OH)2 ↔ ZnO + H2O
Cathode: 2 NiO(OH) + 2 H2O + 2 e− ↔ 2 Ni(OH)2 + 2 OH−
Overall reaction: Zn + 2 NiO(OH) + H2O ↔ ZnO + 2 Ni(OH)2
Cell Voltage: 1.65 V
Silver Zinc Batteries
Anode: Zn + 2 OH− → Zn(OH)2 + 2 e−
Electrolyte: 2 H2O → 2 H+ + 2 OH−
Cathode: Ag2O(s) + 2 H+ + 2 e− → 2 Ag(s) + H2O
Overall reaction: Zn + H2O + Ag2O → Zn(OH)2 + 2 Ag(s)
Cell Voltage: 1.56 V
Uses: button cells
Recent Research
§ Researchers from the University of Maryland and the National Institute of Standards and Technology, have created a water-based zinc battery that is simultaneously powerful, rechargeable and intrinsically safe.
§ The hybrid Zn–Li battery (Zn/LiMn2O4) delivers an excellent cycle performance (energy density 180 W h kg–1 ), with an 85% capacity retained after 4,000 cycles
§ The more challenging Zn/O2 system delivers an unprecedented high energy density of 300 W h kg–1 for 200 cycles §They used Zinc(II) Bis(trifluoromethanesulfonyl)imide + Lithium bis(trifluoromethanesulfonyl)imide (1 m Zn(TFSI)2+ 20 m LiTFSI) as electrolyte.
§ Researchers reported a vanadium oxide bronze pillared by interlayer Zn2+ ions and water (Zn0.25V2O5⋅nH2O), as the cathode for a Zn cell. § The Zn cell offers an energy density of ∼450 Wh l−1 and exhibits a capacity retention of more than 80% over 1,000 cycles, with no dendrite formation at the Zn electrode. The achieved capacity up to 300 mAhg−1
Advantages of Zinc Energy
- Excellent combination of physical and electrochemical properties
- High specific energy and power density. qGood reducing agent with a high theoretical capacity
- One of the most stable metals in aqueous electrolyte solutions
- Zinc-air, Nickel-zinc, and silver-zinc batteries are rechargeable
- Zero-emission, Sustainable, and recyclable
Reference:
1. Fei Wang, Oleg Borodin, Tao Gao, Xiulin Fan, Wei Sun, Fudong Han, Antonio Faraone, Joseph A Dura, Kang Xu & Chunsheng Wang. Nature Materials, 2018, 17, 543–549
2. Kundu, Dipan; Adams, Brian D.; Duffort, Victor; Vajargah, Shahrzad Hosseini; Nazar, Linda F. Nature Energy, 2016, 1, Article number: 16119
Best Zinc Mining Stocks to Buy and Hold forever
I have mentioned the production of zinc by the major miners all over the world. However, personally I like the stocks that mine zinc with other metals also. Below is the list of stocks I recommend to buy and hold for your retirement portfolio. The best part is that they regularly pay dividends and are less affected by the pandemic like COVID-19 or global crisis. Although it is natural to fluctuate their price with broader commodity market.
- Southern Copper Corporation (SCCO)
- BHP Billiton (BHP & BBL)
- Rio Tinto (RIO)
Trade all your favorite stocks, ETF, future, options & cryptocurrency for free of cost. Also, get free stocks of up to $1600 when you sign up and deposit only $100.
Do you know that you can save money every day from the service you like or the items you will purchase? I have summarized all the savings ideas in one single post. Please check out how much you can save today.

