Wastewater is characterized by their physical, chemical and biological characteristics.
Physical Characteristics: Color, Odor, Dissolved oxygen (D.O), Insoluble substances (suspended solids, settleable solids), Corrosive properties, Radioactivity, Temperature, Foamability etc.
Chemical characteristics: Chemical oxygen demand (C.O.D), pH, Acidity or Alkalinity, Hardness, Total carbon, Total dissolved solids, chlorine demand, trace elements & ions, Surfactants, phenols, hydrocarbons, oils and greases etc.
Biological Characteristics: Biochemical oxygen demand (B.O.D), presence of pathogenic bacteria, aquatic organisms, plants and other life forms those are harmful and toxic to man.
Actual methods used for the treatment of a waste depend upon the characteristics of the particular waste. Before choosing particular methods test for wastewater quality indicators in laboratory is done to assess suitability of wastewater for disposal or re-use.
BOD can be used as a gauge of the effectiveness of wastewater treatment plants.

Generally, when BOD levels are high, there is a decline in DO levels. This is because the demand for oxygen by the bacteria is high and they are taking that oxygen from the oxygen dissolved in the water.
Why treatment of water is necessary?
Common Water Pollutants in Surface Water

1.Inorganic Pollutants
2.Organic Pollutants
3.Suspended Solids and Sediments
4. Human Waste
5.Bacteria
6.Pathogens

Wastewater Treatment
The treatment of water can be classified into three categories, Ø Treatment of raw water for drinking purposes Ø Treatment of raw water specialized industrial applications. Ø Treatment of waste water to make it acceptable for release or reuse. v Water that has to be used for domestic purposes should be thoroughly disinfected to eliminate disease causing microorganisms but can contain appreciable amounts of dissolved salts such as calcium and magnesium. v vThe water that has to be used for industrial purposes must be soft to prevent scale formation in boilers but can contain microorganisms.
Although most of the physical and chemical processes used to treat water involve similar phenomena, the method and degree of water treatment are site specific. These processes, which consist of series of unit operations, are applied in different combinations and sequences depending upon the prevailing situations of influent concentration, composition and specifications of the effluent.
Municipal Water Treatment For Raw Water
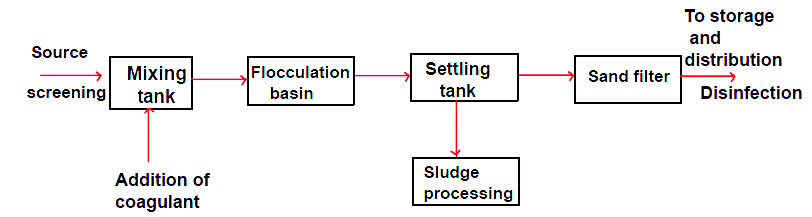
Screening to remove relatively large floating and suspended debris.
Mixing the matter with chemicals that encourage suspended solids to coagulate into larger particles that will more easily settle.
Flocculation, which is the process of gently mixing the water and coagulant, allowing the formation of large floc.
Sedimentation in which the flow is slowed enough so that gravity will cause the floc to settle.
Sludge processing where the mixture of solids and liquids collected from the settling tank are dewatered and disposed of.
Disinfection of the liquid effluent to ensure that the water is free from harmful pathogens.
Treatment Of Raw Water For Industrial Use
The following factors must be taken into account for designing and operating an industrial water treatment facility.
• Quantity of Water requirement
• Quantity and quality of available water sources
• Successive uses for applications requiring progressively lower water quality
• Water recycle
• Discharge standards.
The basic external treatment that is usually applied to the entire water supply is aeration, filtration, and clarification to remove materials from water such as suspended or dissolved solids, hardness and dissolved gases from water that may cause problems. After this basic treatment, the water may be divided into different streams, some to be used without further treatment and the rest to be treated for specific applications.
Internal treatment is designed to modify the properties of water for specific applications. Examples of the internal treatment include the following:
• Addition of either hydrazine or sulfite to remove dissolved oxygen.
• Prevention of formation of calcium deposits by the addition of chelating agents to bind the dissolved calcium.
• Removal of calcium by the addition of precipitants such as phosphate .
• Treatment with dispersants to inhibit scale formation.
• Prevention of corrosion by the addition of inhibitors. • pH adjustment.
Wastewater Treatment

Available wastewater treatment processes can be broadly classified as physical, chemical or biological.
• Physical processes principally comprise screening, sedimentation, floatation and filtration.
• Chemical processes utilize the chemical properties of the impurities of the added reagents. Commonly used chemical processes are precipitation, coagulation and disinfection.
• Other physical and chemical processes such as air stripping, carbon adsorption, oxidation and reduction, ion-exchange and membrane processes like reverse osmosis and electrodialysis are also important in certain cases.
•Biological processes utilize biochemical reactions; typical examples are biofiltration and activated sludge process.
These processes are usually grouped as primary treatment, the secondary treatment, and the tertiary or the advanced waste treatment.
• Primary treatment removes identifiable suspended solids and floating matter.
• In the secondary treatment which is also known as biological treatment, organic matter that is soluble or in the colloidal form is removed.
• Advance waste treatment involves physical, chemical or biological processes or their various combinations depending on the impurities to be removed. These processes are employed to remove residual soluble non-biodegradable organic compounds including surfactants, inorganic nutrients and salts, trace contaminants of various types, and dissolved inorganic salts. The advanced waste treatment processes are expensive, and are used only when water produced is required to be of higher quality than that by secondary treatment.
Primary treatment of wastewater
Primary treatment comprises of-
•pretreatment step and
•sedimentation step
Pretreatment:
It consists of screening and grit removal. Screening removes or reduces the size of trash and large solids that get in to sewage system. These solids are collected on screens and scraped off for subsequent disposal. Comminuting devices shred and grind solids in the sewage. Particle size may be reduced to the extent that the particles can be returned to the sewage flow. After screening, the waste water is allowed to enter a grit chamber for the removal of inorganic grit consisting of sand, gravels and pebbles . Grit chambers are provided to protect pumps from abrasion (ঘর্ষণ) and to reduce the formation of heavy deposits in pipes and channels. Grit is normally allowed to settle in a tank under conditions of low flow velocity and it is then scraped mechanically from the bottom of the tank.
Primary sedimentation:
Primary sedimentation removes both the settleable and floatable solids.
Also the flocculant particles which tend to aggregate will be allowed to settle by the addition of chemicals (iron salts, lime and alum). The material that floats in the primary settling basin is known collectively as grease. Normally some of the grease settles with the sludge and floats to the surface, which can be removed by skimming .
This process of flocculant settling takes place when the settling velocity of the particles increases due to coalescence with other particles. This type of phenomenon is clearly observed in primary clarifiers. The opportunity for coalescence increases with increase in bed depth, and as a result the particle removal efficiency depends on both the overflow and bed depth.
Secondary treatment for municipal wastewater
In secondary or biological treatment, oxygen supplied to the bacteria is consumed under controlled conditions so that most of the BOD is removed in the treatment plant rather than in the water course. Thus, the principal requirements of a biological waste treatment process are an adequate amount of bacteria that feed on the organic material present in waste water, oxygen and some means of achieving contact between the bacteria and the organics.
Two of the most commonly used systems for the biological waste treatment are the biological film system (trickling filters) and the activated sludge system. In the biological-film system the waste water is brought into contact with a mixed microbial population in the form of a film of slime attached to the surface of a solid support medium whereas in the activated sludge system the waste water is brought in contact with a diverse group of microorganisms in the form of a flocculant suspension in an aerated tank. In both cases the organic matter is metabolised to more stable inorganic forms.
The most popular means of treating domestic sewage has been the biological film
system because of its ease of operation. However the activated sludge process can be more reliably be handled when handling large volumes of waste water, and a high degree of treatment is achieved.
Trickling filters
Conventional trickling filters normally consist of a rock bed, 1 to 3 meters in depth, with enough opening between the rocks to allow air to circulate easily . The influent is sprinkled over a bed of packing which is coated with a biological slime. As the liquid trickles over the packing, oxygen and the dissolved organic matter diffuse into the film to be metabolized by the microorganisms in the slime layer. End products such as CO2, NO3– , etc., diffuse back, out of the film and appear in the filter effluent. Milk processing, paper mills and pharmaceuticals wastes are among those treated by trickling filters. Like all biological units trickling filters are affected by temperature; therefore cold weather slows down the biological activity in the filter.

Rotating biological contactor
Trickling filters as discussed previously are examples of devices that rely on microorganisms that grow on the surface of the rocks, plastic or other media. A variation of this attached growth approach is provided by the rotating biological contactactor (RBC). An RBC consists of a series of closely spaced, circular, plastic disks, that are typically 3.6 m in diameter and attached to a rotating horizontal shaft. The bottom of 40% of each disc is submerged in a tank containing the waste water to be treated. The biomass film that grows on the surface of the disks moves into and out of the waste water as RBC rotates. While the microorganisms are submerged in waste water, they absorb organics; while they were rotated out of waste water, they are supplied with needed oxygen. By placing modular RBC units in series, treatment levels that exceed the conventional secondary treatment can be achieved. They are easier to operate under varying load conditions than trickling filters, since it is easier to keep the solid medium wet at all times.
Activated sludge process :
The most versatile and effective of all the waste treatment processes is the activated sludge process. The essential features of the process are: an aeration tank where the organic matter is brought into intimate contact with the sludge from the secondary clarifier. This sludge is heavily laden with microorganisms which are in an active state of growth.
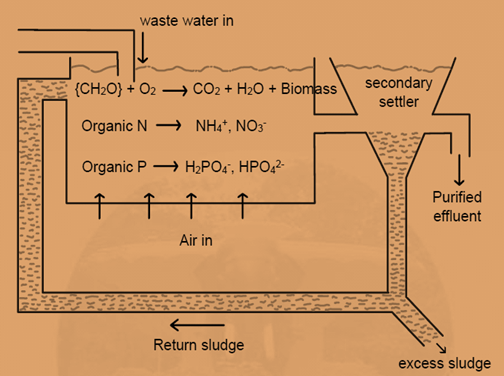
Air is introduced into the tank, either in the form of bubbles through diffusers or by surface aerators. The microorganisms utilize oxygen in the air and convert the organic matter containing N and P into stabilized, low energy compounds such as NO3– , SO42- ,NH4+ ,H2PO4– , HPO42- , CO2, H2O and synthesize new bacterial cells. The effluent from the aeration tank containing the flocculent biomass, known as the sludge, is separated in a settling tank, sometimes called a secondary settler or clarifier.
These solids settle out in the settler and a fraction of them is discarded. Part of the solids is recycled as return sludge to the head of the aeration tank and comes into contact with fresh sewage. The combination of high concentration of “hungry” cells in the return sludge and a rich food source in the influent sewage provides optimum conditions for waste degradation.
(1) In the activated sludge process, BOD is removed by two path ways. Organic matter is oxidized in the course of providing energy for the metabolic processes of the microorganism, and
(2) Synthesis and incorporation of organic matter into cell mass. In the first pathway, carbon is removed in the gaseous form as CO2. The second pathway provides for removal of carbon as a solid in biomass. That portion of the carbon converted to CO2 is vented to the atmosphere and does not present a disposal problem. What remains to be disposed of is a mixture of solids and water called sludge. The collection, processing and disposal of sludge can be the most costly and complex aspect of waste water treatment. The concentration of solids in the primary sewage sludge is about 5% ; in the activated sludge it is less than 1% and the sludge from trickling filters has about 2% solids. This means that the sludge is composed almost entirely of water and volume reduction is the key to economic disposal. In addition to reducing its water content, the sludge must be stabilized so that its biological activity and tendency towards putrefaction are reduced drastically.
The significant processes that occur in biological waste treatment:
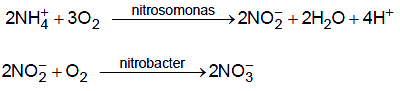
The significant process that occurs during biological waste treatment is nitrification. In this process, ammonium ion is oxidised, under appropriate conditions, first to nitrite by Nitrosomonas bacteria .
2NH4+ + 3O2 → 4H+ +NO2− + 2H2O
then to nitrate by Nitrobacter:
2NO2− + O 2 → 2NO3−
The above reactions occur in the aeration tank of the activated sludge plant and are favored in general by long retention times, low organic loadings, large amounts of suspended solids, and high temperatures. The following denitrification will occur by the action of pseudomonas, in an oxygen deficient settler.
4NO3 − + 5{CH2O} + 4H+ → 2N2(g) +5CO2(g) +7H2O
This causes bubbles to form on the sludge floc, thus making it so buoyant that it floats on the top. This prevents settling of the sludge and increases the organic load in the receiving waters. Under the appropriate conditions, however, advantage can be taken of this phenomenon to remove nutrient nitrogen from water.
Activated sludge treatment produces more microorganisms than necessary and if the microorganisms are not removed, their concentration will soon increase and clog the system with solids. Some of the microorganisms must therefore be wasted and the disposal of such waste activated sludge is one of the most difficult aspects of waste treatment as explained previously.
Oxidation ponds:
Oxidation ponds are large, shallow typically 1-2m deep, where raw or partially treated sewage is decomposed by microorganisms. The conditions are similar to those that prevail in an eutrophic lake. The ponds can be designed to maintain aerobic conditions throughout, but more often the decomposition taking place near the surface is aerobic, while that near the bottom is anaerobic.

Such ponds having a mixture of aerobic and anaerobic conditions, are called facultative (in biology it means-able to exist under more than one set of conditions) ponds. The oxygen required for aerobic decomposition is derived from surface aeration and algal photosynthesis; deeper ponds called lagoons, are mechanically aerated. The reactions taking place in a facultative pond is shown in above figure.
Oxidation ponds, can be designed to provide complete treatment to raw sewage, but they require good deal of space. These ponds have been used extensively in small communities where land constraints are not so critical. They are easy to build and manage. They accommodate large fluctuations in flow, and they can provide treatment that approaches that of conventional biological systems but at much lower cost. The effluent however may contain undesirable concentrations of algae and, especially in the winter when less oxygen is liberated by photosynthesis which may produce unpleasant odors. They have the disadvantage that the effluent may not meet the EPA secondary treatment requirement of 30 mg/L BOD and suspended solids. However they are simple and effective in destroying pathogenic organisms which make these ponds useful in developing countries. Oxidation ponds are also used to supplement secondary treatment and in such cases they are called polishing ponds.
Advanced Wastewater Treatment
The effluent from a typical secondary treatment plant still contains 20-40 mg/L BOD which may be objectionable in some streams. Suspended solids, in addition to contributing to BOD, may settle on the stream bed and inhibit certain forms of aquatic life. The BOD if discharged into a stream with low flow, can cause damage to aquatic life by reducing the dissolved oxygen content.
In addition the secondary effluent contains significant amounts of plant nutrients and dissolved solids. If the waste water is of industrial origin, it may also contain traces of organic chemicals, heavy metals and other contaminants. So, advance treatment is required to make it usable.
Different methods are used in advanced waste treatment to satisfy any of the several specific goals, which include the removal of
(1) suspended solids
(2) BOD
(3) plant nutrients
(4) dissolved solids and
(5) toxic substances.
These methods may be introduced at any stage of the total treatment process as in the case of industrial waterways or may be used for complete removal of pollutants after secondary treatment.
Removal Of Solids:
Removal of Suspended Solids:
This treatment implies the removal of those materials that have been carried over from a secondary treatment settler. Many methods were proposed of which two methods were commonly used. The two methods are microstaining and chemical coagulation followed by settling and mixed media filtration.
Microstraining:
It is a special type of filtration procedure which makes use of filters woven from stainless steel wires with opening only 60-70 µm across to remove very small particles. High flow rates and low back pressures are normally achieved.
Coagulation and flocculation:
The object of coagulation is to alter these particles in such a way as to allow them to adhere to each other. Most colloids of interest in water treatment remain suspended in solution because they have a net negative surface charge that causes the particles to repel each other. The intended action of the coagulant is to neutralize that charge, allowing the particles to come together to form larger particles that can be more easily removed from the raw water.
The usual coagulant is alum [Al2(SO4)2•18H2O], though FeCl3, FeSO4 and other coagulants, such as polyelectrolytes, can be used. Alum when added to water, the aluminium in this salt hydrolyses by reactions that consume alkalinity in the water such as:
[Al(H2 O)6 ]3+ + 3HCO3− → Al(OH)3(s) +3CO2 + 6H2O
The gelatinous hydroxide thus formed carries suspended material with it as it settles. In addition, however, it is likely that positively charged hydroxyl-bridged dimers such as

and higher polymers are formed which interact specifically with colloidal particles, bringing about coagulation. Metal ions in coagulants also react with virus proteins and destroy upto 99% of the virus in water.
Anhydrous ion (III) sulphate can also act as effective coagulant similar to aluminium sulfate. An advantage with iron (III) sulfate is that it works over a wide range of pH.
Filtration:
If properly formed, the addition of chemicals for promoting coagulation and flocculation can remove both suspended and colloidal solids. After the flocs are formed, the solution is led to a settling tank where the flocs are allowed to settle. While most of the flocculated material is removed in the settling tank, some floc do not settle. These flocs are removed by the filtration process, which is usually carried out using beds of porous media such as sand or coal. The current trend is to use a mixed -media filter which consists of fine garnet in the bottom layer, silica sand in the middle layer and coarse coal in the top layer which reduces clogging.
Removal of dissolved solids from wastewater
The dissolved solids are of both organic and inorganic types. A number of methods have been investigated for the removal of inorganic constituents from wastewater. Three methods which are finding wide application in advanced waste treatment are
1.ion-exchange,
2.electrodialysis and
3.reverse osmosis.
For the removal of soluble organics from waste water the most commonly used method
is adsorption on activated carbon. Solvent extraction is also used to recover certain organic
chemicals like phenol and amines from industrial waste waters.
Ion exchange treatment of Wastewater
This technique has been used extensively to remove hardness, and iron and manganese salts in drinking water supplies. It has also been used selectively to remove specific impurities and to recover valuable trace metals like chromium, nickel, copper, lead and cadmium from industrial waste discharges. The process takes advantage of the ability of certain natural and synthetic materials to exchange one of their ions.
A number of naturally occuring minerals have ion exchange properties. Among them the notable ones are aluminium silicate minerals, which are called zeolites. Synthetic zeolites have been prepared using solutions of sodium silicate and sodium aluminate. Alternatively synthetic ion-exchange resins composed of organic polymer with attached functional groups such as -SO3– H+ (strongly acidic cation exchange resins), or – COO– H+ (weakly acidic cation exchange resins or -N+(CH3)3OH– (strongly basic anion exchange resins) can be used.
In the water softening process, the hardness producing elements such as calcium and magnesium are replaced by sodium ions. A cation exchange resin in sodium form is normally used. The water-softening capability of cation exchange can be seen when sodium ion in the resin is exchanged for calcium ion in solution.
2 Res SO3− Na+ + Ca2+ → (Res SO3−)2 Ca2+ + 2Na+
(where “Res” represents resin phase)
The product water thus has high sodium content, which is not likely to be troublesome unless the original water is very hard. When the exchanger is saturated, it has to be regenerated to allow reuse of expensive resin. Regeneration can be achieved by sodium chloride solution which removes Ca2+ and Mg2+ ions from the resin.
(Res SO3− )2 Ca2 + + 2Na+ + 2Cl− → 2Na+(Res SO3−) +Ca2+ + 2Cl−
Since for regeneration large amounts of NaCl has to be used, appreciable amounts of sodium chloride can be introduced into sewage by this route. This problem can be overcome by using weakly acidic cation exchange resin such ResCOO–H+. These cation exchangers having -COOH as functional group are useful for removing alkalinity along with hardness. Alkalinity is generally manifested by bicarbonate ion. This ion is sufficiently basic to neutralise the acid of weak cation exchange. Another advantage with these resins is that these can be regenerated almost stoichiometrically with dilute strong acid, thus avoiding pollution problem caused by excess NaCl. This technique has also been successfully applied to the recovery of chromate from waste water in pigment manufacturing.
The removal of inorganic solute is essential for complete water recycling. The effluent from secondary waste treatment contains 300-400 mg/L more dissolved inorganic material than does municipal water. The removal of these bulk inorganics can be efficiently done by reverse osmosis and electrodialysis.
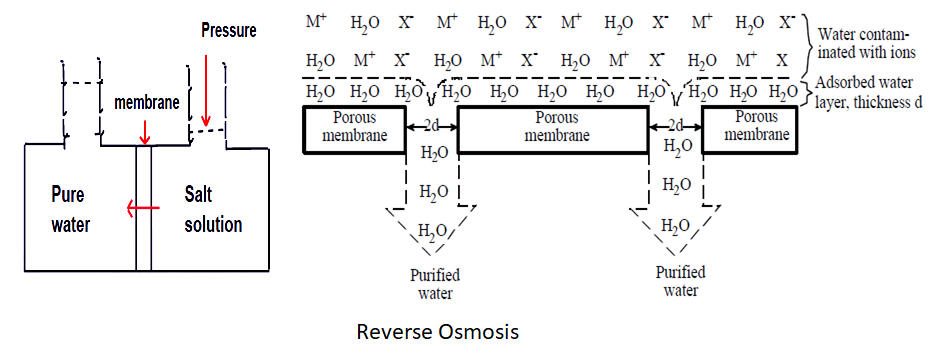
Reverse osmosis treatment of wastewater
In the reverse osmosis process, demineralised water is produced by forcing water through semi-permeable membranes at high pressure. In ordinary osmosis, if a vessel is divided by a semi-permeable membrane and one compartment is filled with water and other with concentrated salt solution, water diffused through the membrane towards the compartment containing salt solution until the difference in water levels on the two sides of the membrane creates a sufficient pressure to counteract the original water flow. The difference in levels represents the osmotic pressure of the solution.
The process can be reversed by applying sufficient pressure to the concentrated solution to overcome the osmotic pressure force the net flow of water through the membrane towards the dilute phase. The solute concentration (impurity) builds up on one side of the membrane while relatively pure water passes through the membrane . In order to obtain adequate solvent (water) flux through the membrane, pressures of the order of 4000 to 7000 kN/m2 are required. Figure (a) represents the principle of operation of the reverse osmosis unit.
Electrodialysis treatment of Wastewater
Electrodialysis uses ion-selective membranes and an electrical potential difference to separate anions and cations in solution
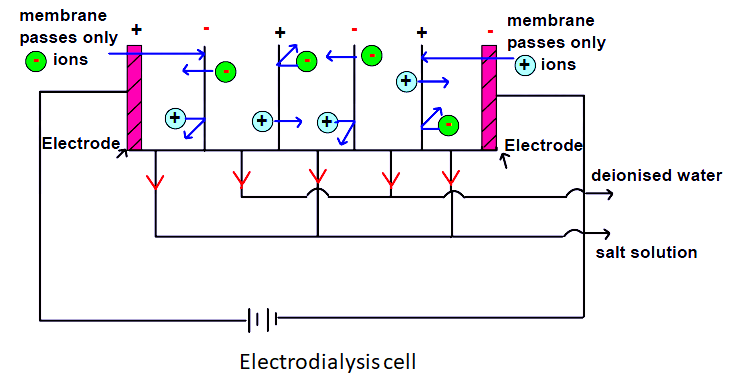
In the past electrodialysis was most often used for purifying brackish water, but it is now finding a role in hazardous waste treatment. Metal salts from plating rinses are sometimes removed in this way.
Figure shows a simple dialysis cell in which waste water may be deionised. As shown in the figure two types of membranes (anionic and cationic) are arranged alternatively to form many compartments between the electrodes placed at the two ends. When the voltage is applied across the cell containing mineralised water, the anions migrate to the positive electrode and the cations migrate to the negative electrode. This causes solution in alternate compartments to become more concentrated while that in the remaining becomes more dilute. The electric power requirement is proportional to the number of ions removed from the water.
In the electrodialysis process, organic molecules are not removed and they can collect on and clog the membranes. Another disadvantage of this method is that it still leaves concentrated wastewater to be disposed of by some appropriate scheme. The process does not require any chemical additives and has low energy requirements and as such it can be an economically feasible means of demineralisation.
Removal of nitrogen from wastewater
Nitrogen compounds may be removed in waste water in two ways. Even after secondary treatment, most of nitrogen exists as ammonia. Increasing the pH produces the reaction,
NH4+ + OH− →NH3 ↑ +H2O
These reactions are slow and require long retention times in the aeration tank as well as sufficient DO. If the flow rate is too high, the slow-growing microorganisms are washed out of the aeration tank.
Once the ammonia has been oxidised to nitrate, it may be reduced by anaerobic bacteria like pseudomonas. This denitrification requires a source of carbon and methanol is often used for that purpose.
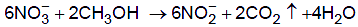
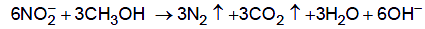
Phosphate removal from wastewater (chemical treatment):
Phosphate may be removed chemically or biologically. The most popular chemical methods use lime, Ca(OH)2 and alum, Al2(SO4)3.Under alkaline conditions, the calcium will combine with phosphate to form calcium hydroxyapatite, a white insoluble precipitate that is settled out and removed from waste water. Insoluble calcium carbonate is also formed and removed.
5 Ca(OH)2 + 3HPO24− → Ca5OH(PO4 )3 ↓ +3H2O + 6OH−
The aluminium ion from alum precipitates as very slightly soluble aluminium phosphate,
Al3 + + PO34− → AlPO4 ↓
and also forms aluminium hydroxide.
Al3+ + 3OH− → Al(OH)3 ↓
which forms sticky flocs that help to settle out phosphates.
Phosphate removal from wastewater (biological treatment)
Biological phosphorous removal does not require the addition of chemicals. In this process, the aeration tank in the activated sludge system is subdivided into zones, some of which are not aerated. In these zones, the aerobic microorganisms become solely stressed because of the lack of oxygen. If these microorganisms are then transferred to an aerated zone, they try to make up for lost time and assimilate organic matter (as well as phosphorous) at a rate much higher than they ordinarily would. Once the microorganisms have adsorbed the phosphorous, they are removed as waste activated sludge, thus carrying with them high concentrations of phosphorous. Using such sequencing of non aerated and aerated zones, it is possible to remove as much as 90% of the phosphorous.
Removal of dissolved organic compounds from wastewater
Adsorption:
One of the most commonly used techniques for removing organics involves the process of adsorption, which is the physical adhesion of chemicals on to the surface of the solid. The effectiveness of the adsorbent is directly related to the amount of surface area available to attract the particles of contaminant. The most commonly used adsorbent is a very porous matrix of granular activated carbon, which has an enormous surface area (~ 1000 m2/g). Adsorption on activated carbon is perhaps the most economical and technically attractive method available for removing soluble organics such as phenols, chlorinated hydrocarbons, surfactants, and colour and odour producing substances from waste water.
Granular activated carbon treatment systems consist of a series of large vessels partially filled with adsorbent. Contaminated water enters the top of each vessel, trickles down through granulated activated carbon, and is released at the bottom. After a period of time, the carbon filter becomes clogged with adsorbed contaminants and must be either replaced or regenerated. Regeneration of the carbon is accomplished by heating it to 950oC in a steam air atmosphere. This process oxidises surface, with an approximately 10% loss of carbon.
Synthetic organic polymers such as Amberlite XAD-4 have hydrophobic surfaces and are quite useful in removing relatively insoluble organic compounds such as chlorinated pesticides. These absorbents are readily regenerated by solvents such as isopropanol and acetone.
Sludge treatment and disposal from sewage water
Both primary and secondary sewage treatments involve settling of particulate matter, and thus produce sludge. The concentration of solids in the primary sewage sludge is about 5%; the activated sludge contains about 1%; and the sludge from trickling filters has about 2% solids. The sludge must be stabilized so that its biological activity and tendency towards putrefaction are reduced drastically.
The sludge is concentrated by gravity settling and floatation. After concentration the sludge is subjected to anaerobic digestion in a digester in which the organic content of the sludge decomposes to give mainly methane and carbondioxide and at the same time the bound water is released from the sludge.
The sludge is then conditioned to improve its dewatering characteristics by adding chemicals like iron salts and polyelectrolytes. These chemicals bind the sludge particles together and encourage the release of water. The sludge is then heated under pressure and after a period of time the gel structure of the sludge breaks down so that the water is released. The thickened sludge is then dewatered for efficient handling and disposal. The dewatering is accomplished by mechanical methods, the most common being centrifugation and filtration. The dewatered sludge is then subjected to oxidation to reduce the organic content, with the consequent destruction of bacteria and a significant reduction in their volumes. Incineration and wet oxidation are the two common methods employed for oxidation.
Several methods are employed for the ultimate disposal of sludge. The wet digested sludge may be sprayed on to a cropland where it functions as fertiliser. Dried sludge may be used a land fill or soil conditioner. Wet or partially dewatered sludge or ash from incineration may be transported from the shore to dumping grounds at sea. The potential drawback to the use of sewage sludge as fertiliser in agricultural fields is the presence of both organic and inorganic toxic compounds. The former compounds are oxidation-resistant organic substances, such as organochlorine species which become bound in the organic matrix of the sludge. The inorganic toxicants are represented by heavy metals, mainly arsenic, cadmium , lead, mercury and zinc. These metals can be taken up by crops and introduced into the food chains or leached to the ground water.
Disinfection of drinking water
Disinfection, using chemical and physical methods is the final step in drinking water purification. The finished water is disinfected often with chlorine. It kills the remaining microorganisms in the water, some of which will be pathogenic. It is a very efficient oxidising, bleaching and disinfecting agent. In water chlorine reacts as follows:
Cl2 +H2O →H+ +Cl− +HOCl
The hypochlorous acid (HOCl) is the prime disinfecting agent.
HOCl← →H+ +OCl−
Together, HOCl and OCl– (Hypochlorite ion) are called the free available chorine.
A principal advantage of chlorination over other forms of disinfection is that a chlorine residual is created that can protect the treated water after leaving the treatment plant. This guards against possible contamination that might occur in water distribution system. To increase the lifetime of the residual, some systems add ammonia to the treated water, forming chloramines.
NH+4 + HOCl →NH2Cl (monochloramine) +H2O +H+
NH2 Cl + HOCl →NHCl2(dichloramine) +H2O
NHCl2 + HOCl →NCl3(trichloramine) +H2O
Chloramines, although they are less effective as oxidants than HOCl, are more persistent. Residual chlorine that exists as chloramine is referred to as combined available chlorine. Chlorine may have adverse secondary effects. It has the potential to combine with trace amounts of organic substances to form trihalomethanes (THMs) such as the carcinogen chloroform. Some studies have shown an association between bladder and rectal cancer and consumption of chlorinated drinking water. One approach to reducing THMs is to remove more of the organics before any chlorination takes place, which can be accomplished by adsorption on activated carbon.
The problem faced with the formation of THMs has spurred interest in alternatives to chlorination as the preferred method of disinfection. Alternative disinfectants include chlorine dioxide and ozone. Chlorine dioxide (ClO2) is a potent bactericide and viricide and it does not form a residual capable of protecting water in the distribution system. However, there is concern for certain toxic chlorate and chlorite substances that it may create, and it is a very costly method of disinfection. Ozonation involves the passage of ozone (O3) through water. Ozone is a very powerful disinfectant that is even more effective against cysts and viruses than chlorine, and it has the added advantage of having no taste or odour problems. Unfortunately, the disinfective power of ozone is limited by its relatively low solubility in water. It is more expensive and difficult to store as well.

