What is Dissolved Oxygen?
The amount of free, non-compound oxygen ( as O2) dissolved in a body of water such as a ocean, sea, lake, river, or stream etc. Non-compound oxygen, or free oxygen (O2), is oxygen that is not bonded to any other element. Dissolved oxygen is the presence of these free O2 molecules within water.The bonded oxygen molecule in water (H2O) is in a compound and does not count toward dissolved oxygen levels.
Oxygen enters the water through two natural processes:
(1) diffusion from the atmosphere and
(2) photosynthesis by aquatic plants.
The mixing of surface waters by wind and waves increases the rate at which oxygen from the air can be dissolved or absorbed into the water
.
| Carbon dioxide | + | Water | ————–> | Oxygen | + | Carbon-rich foods |
| CO2 | H2O | O2 | C6H12O6 |
From the air, oxygen can slowly diffuse across the water’s surface from the surrounding atmosphere, or be mixed in quickly through aeration, whether natural or man-made 7. The aeration of water can be caused by wind (creating waves), rapids, waterfalls, ground water discharge or other forms of running water. Man-made causes of aeration vary from an aquarium air pump to a hand-turned waterwheel to a large dam.
Dissolved Oxygen Saturation
In a stable body of water with no stratification, dissolved oxygen will remain at 100% air saturation. 100% air saturation means that the water is holding as many dissolved gas molecules as it can in equilibrium. At equilibrium, the percentage of each gas in the water would be equivalent to the percentage of that gas in the atmosphere – i.e. its partial pressure ¹³. The water will slowly absorb oxygen and other gasses from the atmosphere until it reaches equilibrium at complete saturation 10. This process is sped up by wind-driven waves and other sources of aeration.
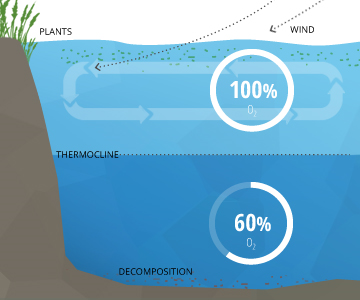
In deeper waters, DO can remain below 100% due to the respiration of aquatic organisms and microbial decomposition. These deeper levels of water often do not reach 100% air saturation equilibrium because they are not shallow enough to be affected by the waves and photosynthesis at the surface ³. This water is below an invisible boundary called the thermocline (the depth at which water temperature begins to decline)¹¹.
DO levels are influenced by temperature, pressure and salinity. The solubility of oxygen, or its ability to dissolve in water, decreases as the water’s temperature and salinity increase. DO levels in an estuary ( এসচুয়ারি/ US:চুয়েরি, নদীর মোহনা) also vary seasonally, with the lowest levels occurring during the late summer months when temperatures are highest.
First, the solubility of oxygen decreases as temperature increases ¹. This means that warmer surface water requires less dissolved oxygen to reach 100% air saturation than does deeper, cooler water. For example, at sea level (1 atm or 760 mmHg) and 4°C (39°F), 100% air-saturated water would hold 10.92 mg/L of dissolved oxygen. ³ But if the temperature were raised to room temperature, 21°C (70°F), there would only be 8.68 mg/L DO at 100% air saturation ³.
Second dissolved oxygen decreases exponentially as salt levels increase ¹. That is why, at the same pressure and temperature, saltwater holds about 20% less dissolved oxygen than freshwater
Third Dissolved oxygen will increase as pressure increases . This is true of both atmospheric and hydrostatic pressures. Water at lower altitudes can hold more dissolved oxygen than water at higher altitudes. at greater hydrostatic pressures, water can hold more dissolved oxygen without it escaping ¹. Gas saturation decreases by 10% per meter increase in depth due to hydrostatic pressure ¹². This means that if the concentration of dissolved oxygen is at 100% air saturation at the surface, it would only be at 70% air saturation three meters below the surface.
Adequate dissolved oxygen is important for good water quality and necessary to all forms of life. Dissolved oxygen levels that drop below 5.0 mg/L cause stress to aquatic life. Lower concentrations cause greater stress . Oxygen levels that go below 1-2 mg/L for a few hours may result in large fish kills.
What is BOD (Biochemical Oxygen Demand)?
BOD (Biochemical Oxygen Demand), also often referred to as biological oxygen demand, is a test performed to measure the amount of dissolved oxygen needed by aerobic microorganism to break down organic materials present in a certain water sample. The BOD test involves taking an initial dissolved oxygen (DO) reading and a second reading after five days of incubation at 20oC. For this reason, this test is often written as BOD5 for short. The BOD value is most commonly expressed in milligrams of oxygen consumed per litre of sample during 5 days of incubation at 20 °C.
When there is an abundance of bacteria and organic materials, the bacteria will take in oxygen in order to breakdown these molecules. If bacteria are taking in large amounts of oxygen, this will have a detrimental effect on the surrounding ecosystem. On the contrary, when there are low levels of organic waste in the water, there are fewer bacteria present, the BOD will be lower and the dissolved oxygen levels higher.
BOD can be used as a gauge of the effectiveness of wastewater treatment plants. In wastewater treatment plants, they often calculate the percentage removal of BOD to determine the efficiency of the treatment process. For this reason, BOD is sometimes referred to as a water contaminant.
What is COD (Chemical Oxygen Demand)?
The chemical oxygen demand (COD) test is commonly used to indirectly measure the amount of organic compounds in water. In these methods, a fixed volume with a known excess amount of the oxidant (KMnO4, K2Cr2O7) is added to a sample of the solution being analyzed. An exactly known excess potassium dichromate Cr2O72- and Sulphuric acid is mixed to oxidize the organic matter in solution to carbon dioxide and water under acidic conditions. Often, the test also involves a silver compound to encourage oxidation of certain organic compounds and mercury to reduce the interference from oxidation of chloride ions. The sample is then digested for approximately 2 hours at 150°C.. After a refluxing digestion step, the initial concentration of organic substances in the sample is calculated from a titrimetric or spectrophotometric determination of the oxidant still remaining in the sample. The amount of oxygen required is calculated from the quantity of chemical oxidant consumed.
Higher COD levels mean a greater amount of oxidizable organic material in the sample, which will reduce dissolved oxygen (DO) levels. A reduction in DO can lead to anaerobic conditions, which is deleterious to higher aquatic life forms. The COD test is often used as an alternate to BOD due to shorter length of testing time.
Difference between BOD (Biochemical Oxygen Demand) and COD (Chemical Oxygen Demand)
It is important to understand that COD and BOD do not necessarily measure the same types of oxygen consumption. For example, COD does not measure the oxygen-consuming potential associated with certain dissolved organic compounds such as acetate. However, acetate can be metabolized by microorganisms and would therefore be detected in an assay of BOD. In contrast, the oxygen-consuming potential of cellulose is not measured during a short-term BOD assay, but it is measured during a COD test. However, COD is less specific, since it measures everything that can be chemically oxidized, rather than just levels of biologically active organic matter.


Pingback: Marine Chemistry: Marine Resources, Pollutions and effects on Biota – Home of Chemistry Lovers
Pingback: Common Terms in Environmental Chemistry – Home of Chemistry Lovers