Nucleon: The constituent part of a nucleus (i.e. a neutron or proton).
Nuclei: Plural of nucleus (one nucleus, 5 nuclei).
Nuclide: Specific types of atoms are called nuclides. These nuclides are distinguished by a specific atomic number Z, which is the number of protons in that atom, and a specific mass number A.
Scientists believe that quarks and leptons are fundamental constituents of matter. The visible matter consists of atoms, and atoms contain nuclei and electrons. The constituents of atomic nuclei are called nucleons. The two types of nucleons are protons and neutrons. A nucleus containing Z protons and N neutrons (i.e., A = Z + N nucleons) is to be denoted by AZ XN, where X is the chemical symbol of the element with Z protons.
Example: 136C7
Nucleons contain quarks of two types: up and down quarks with some additional dynamical (or sea) quark contributions.
What is quark?
Inspired by James Joyce’s Ulysses: “Three quarks for Muster Mark” Gell-Mann first chose the name “Quark”. Particle physicists believe that Quarks are the elementary particles and the building blocks of protons, neutrons, and other particles. There should be six different kinds of quarks, designated by the letters u (for ‘up’ quark), d (down), s (strange), c (charmed), b (bottom or beauty), and t (top or truth). Quark possesses all fundamental interactions – strong, electromagnetic, weak, and gravitational.
What is lepton?
Lepton is an elementary particle that contributes spin in the matter. The quantum spin of lepton is ½. So, if the spin of the fundamental particles are not ½, can the be lepton? The answer is No. They are called bosons. Interestingly, among the six leptons, three have an electrical charge and the other three do not. I am pretty sure you are familiar with at least one of them, the electron (e–). The muon(μ) and the tau(τ) are also charged. The three neutrinos (ν) are leptons with no electric charge. They contribute very little mass, and hard to find.
Does the nucleus has any force?
Nuclei are in a bound state ( the tendency of particles to remain localized) and stable. It’s not possible without the presence of force. Let’s see among the four fundamental forces (strong, electromagnetic, weak, and gravitational) what suits the Nucleus most. Since gravitational force depends on mass, therefore, it is the weakest among the four fundamental forces and plays no practical role in the nuclear force. Charged particles possess electromagnetic forces. In addition to electromagnetic forces leptons enter into weak interactions. Quarks contribute to all the four fundamental forces in the nucleus.
What is binding energy and how to calculate it?
The energy liberated in the formation of a nucleus from its component nucleons is termed as the binding energy (EB). The binding energy can be measured as
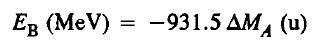
Let’s see an example..
The 42He nucleus is composed of 2 neutrons and 2 protons. The measured mass of the 42He atom is 4.002 603 u. The mass defect is:
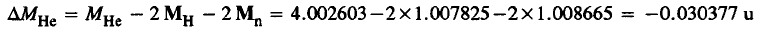
Thus the binding energy for 42He = – 931.5 x -0.030377 = 28.2962 MeV≈ 28.3 MeV
It indicates that to break 42He into its basic component nucleons would require at least 28.3 MeV.
Significance of binding energy per nucleons
Binding Energy per Nucleons (EB/A) signifies the relative stability of nuclei. When you divide the binding energy by the total number of nucleons you will end up with EB/A. The following example will clarify it-
For 42He the value of EB/A is 28.3/4 or 7.1 MeV , where as for 21H it is 1.11.
Clearly, the 42He nucleus is considerably more stable than the 21H nucleus. Hmm sound good…..
What is the most tightly bound nucleus?
At this point, you might be thinking that what is the most tightly bound natural nucleus? Look at the graph of binding energy per nucleon below. It clearly shows maxima at 56Fe. This testifies that 56Fe nuclei are the most tightly bound nuclei. Surprisingly, all the other nuclei want to become like 56Fe. Therefore, nuclei with fewer nucleons can become more tightly bound through fusion, and nuclei with more nucleons can become more tightly bound through the process of fission.
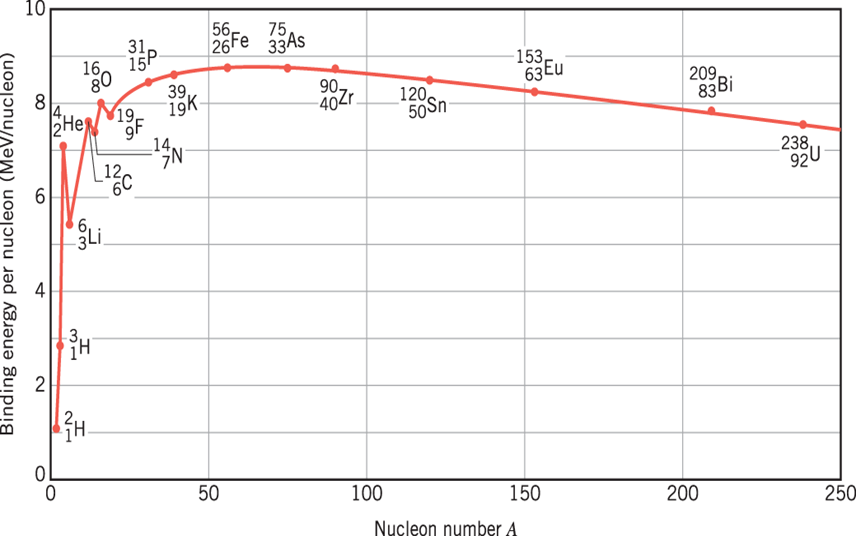
Does the nucleus have a radius?
Rutherford; through his famous scattering experiments proved that the nucleus occupies a very small portion of the total volume of the atom. The best estimate, the radii of nuclei vary from 1/10, 000 to 1/100, 000 of the radii of atoms. The unit of nuclear redii is the femtometer (1 fm = 10 -15 m), also referred to as 1 Fermi. Keep in mind that the atomic sizes are of the order of 100 pm (10-10 m).
The volumes of nuclei (Vn) are directly proportional to the total number of nucleons present, i.e.
Vn α A
Since for a sphere V α R3, where R is the radius of the sphere, for a spherical nucleus
R3 α A
R α A1/3
R = roA1/3
where the nuclear radius constant (ro) can be taken to be 1.2 fm for the “charge radius” and 1.4 fm for the “matter radius.” If someone measures the nuclear radius by scattering high-energy electrons from the nucleus or measures the radius by scattering low-energy ions from the nucleus, the results are slightly different.
Why all the nuclei have the same nuclear density?
The radius of nucleus, R = roA1/3 .
The volume of a spherical nucleus, V = (4/3)πR3
The density of the Nucleus = mass/volume
ρ = M/ (4/3) πR3 [M= mass of nucleus]
= M/ (4/3) π (roA1/3)3 M= A*1.67×10-27 kg since, 1u = 1.67×10-27 kg
= A*1.67×10-27 kg / (4/3) π ro3A
= 3*1.67×10-27 kg / 4π ro3
= 3*1.67×10-27 kg / 4π (1.2×10-15 m)3
= 2.307×1017 kg/m³
In conclusion, all the nuclei have the same nuclear density.
Don’t agree with me!!! Calculate the density of 42He nucleus where ro = 1.2 fm.
What is the magic number of the nuclei?
Nuclei with even numbers of protons and neutrons are more stable than those with odd numbers. In reality, there are “magic numbers” of neutrons and protons which provide more nuclear stability.
The magic numbers:
proton: 2, 8, 20, 28, 50, 82, 114
neutron: 2, 8, 20, 28, 50, 82, 126, 184
Hmm… what happens if both neutron number and proton number match with the magic numbers? They are called “doubly magic”, and are your guess is correct, they are particularly stable.
For example, 4020Ca (Z= 20 and N= 20) and 4820Ca (Z= 20 and N= 28) both have more binding energy than calculated from the Weizsaecker formula.
How nuclides are classified?
1. Stable Nuclides: Nuclides that do not has radioactive decay. Around 264 stable nuclides are reported so far. e.g. 12C, 14N etc.
2.Primary Natural Radionuclides: These are radioactive nuclides but are persisted on earth from the origin of the solar system (yes, you heard it right!) . These radionuclides have very long half-lives. 238U (half-life = 4.47 x 109 years). About 26 primary natural radionuclides are reported to date.
3.Secondary Natural Radionuclides: Natural radionuclides that have been produced by the decay of the primary natural radionuclides. They have too short half-life to survive. As yet 38 of them are identified. e.g. 234Th ( half life = 24.1 days).
4.Induced Natural Radionuclides: These radionuclides are constantly being produced by the action of cosmic rays on the earth’s atmosphere. About 10 induced natural radionuclides are known heretofore. e.g. 3H ( Tritium; half life = 12.3 years).
5. Artificial Radionuclides: You know that man loves thrill and advantage. This gave birth to more than 2000 artificial radionuclides which are insignificant in nature. e.g 60Co, 24Na etc.

